This author assumes absolutely no responsibility for anything you do, as a result of reading this material, or for any other reason. I make no claims as to accuracy. Always assume your own responsibility for research and for your actions. Read all the warnings. Read the articles. Decide what you may be willing and unwilling to put in your head.
DMT from Phalaris
Abbreviations, nomenclature, etc.
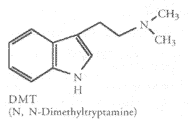
The following alkaloids are discussed and abbreviated as indicated:N,N-Dimethyltryptamine (DMT)
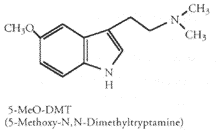
5-Methoxy-N,N-Dimethyltryptamine (5-MeO-DMT)
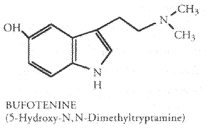
5-Hydroxy-N,N-Dimethyltryptamine (5-OH-DMT or bufotenine).
Phalaris aquatica is also called Phalaris tuberosa. I use P. tuberosa in this text.
The drugs in question
|
|
|
5-MeO-DMT is a close relative of DMT. 5-MeO-DMT is reported to be about 4 times as potent as DMT and is often regarded as preferable to DMT. 5-MeO-DMT has most of the psychedelic qualities as DMT but does not cause visual hallucinations. It follows the same time course.
Bufotenine (5-OH-DMT) is another DMT relative. This compound is vaguely referred to as "noxious" by Jonathan Ott. Apparently 10mg of pure 5-OH-DMT injected is enough to cause "dramatic circulatory crises." There appears to be debate over the psychedelic qualities of bufotenine. However, McLeod & Sitaram, Shulgin, and Fabins & Hawkings all report the presence of psychotomimetic effects. Bufotenine causes anxiety, circulatory distress, skin flushing, and percieved color distortions. Injected doses of 16 mg (over 3 min. IV drip) have been reported. At this dose, the symptoms of mild skin flushing to extreme purple cast appear. Subjects also report a great deal of psychological distress and fear at this dose. Doses of 8 mg produce mild skin flushing and increased anxiety. Doses of 2-4 mg of bufotenine do not produce hallucinogenic effects. The above discussed negative side effects at 16 mg last for approximately 10 minutes. Other side effects reported are sweating, nausea, yellowed vision, and perception of colored spots. So it appears that bufotenine is a nasty drug to be avoided, Not only does it tend to induce panic, it also appears to have the potential for a fatal overdose, although no case studies to this effect have been found for humans.
Other DMT relatives exist, but are not of great importance here. Some are, however, psychoactive.
DMT in the past has only been used by smoking or injecting. Oral use was completely ineffective. It turns out that ancient tribal cultures solved that problem aeons ago, and have been dosing up the whole time. Now that's technology. By combining plants that contained MAOIs (monoamine oxidase inhibitors) and other plants containing DMT, the DMT would become active orally. MAOIs block the destruction of DMT in the digestive track and in the brain. So, with MAOIs, DMT can be eaten and also becomes more potent. MAOIs also increase the potency of smoked DMT. The effect of the orally administered DMT with MOAI lengthens in time and decreases in intensity. Typical plateau period is 10-40 minutes, after a hour delay with low buzz for an hour after the plateau. Great for people who don't like the time investment of most psychedelics.
 MAOIs are reported to work for psilocybin ('shrooms) and mescaline.
Subjective reports are that MAOIs double their potency. I hypothesize that
it will also potentiate lysergic acid (woodrose and morning-glories). The
effect of MAOIs on LSD appears debated in the literature. Some report no
effect, others report significant potentiation. Some have very
enthusiastically insisted that MAOIs double or triple the potency of LSD.
MAOIs are reported to work for psilocybin ('shrooms) and mescaline.
Subjective reports are that MAOIs double their potency. I hypothesize that
it will also potentiate lysergic acid (woodrose and morning-glories). The
effect of MAOIs on LSD appears debated in the literature. Some report no
effect, others report significant potentiation. Some have very
enthusiastically insisted that MAOIs double or triple the potency of LSD.
The ancient solution mentioned above is the ayahuasca potion. This potion has been produced, in one form and name or another, throughout Central America and South America for quite some time. The most commonly referred to potion is made by combining ayahuasca, a jungle vine, and yopo, leaves from a small bush. The ayahuasca provides the MAOIs and the yopo provides DMT. Occasional, mescaline bearing cacti are added. The potion was usually used ceremonial for healing, divining, and teaching. Often there are reports of blue glows and jaguars, a holy animal in many endogenous South American cultures. I have been told that McKenna reports that eskimos given an ayahuasca type potion reported seeing large cats, which, of course, are not arctic animals. I however, have found (from admittedly little reading) McKenna's work to be questionable and less than scientific. However, his reports often do parallel others.
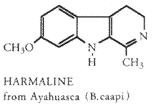 MAOIs are a class of drugs that all do the same thing: prevent the
destruction of monoamines (like DMT). One MAOI is harmaline. Harmaline is
easily obtained. Syrian rue is an excellent source. Three grams of seed,
extracted with the DMT or eaten alone should suffice. Harmaline containing
plants can also be smoked for a more rapid onset. Doses over three grams do
not add more potency. Caution should be used with MAOIs. Large doses are
hallucinogenic in and of themselves. Large doses are unpleasant and
sometimes fatal. (You should also read this list of Foods to Avoid with
MAO-Inhibitors.)
MAOIs are a class of drugs that all do the same thing: prevent the
destruction of monoamines (like DMT). One MAOI is harmaline. Harmaline is
easily obtained. Syrian rue is an excellent source. Three grams of seed,
extracted with the DMT or eaten alone should suffice. Harmaline containing
plants can also be smoked for a more rapid onset. Doses over three grams do
not add more potency. Caution should be used with MAOIs. Large doses are
hallucinogenic in and of themselves. Large doses are unpleasant and
sometimes fatal. (You should also read this list of Foods to Avoid with
MAO-Inhibitors.)
 P. tuberosa |
DANGEROUS COMBINATIONS
READ THIS!! VERY IMPORTANT. IGNORING THIS COULD LEAD TO SERIOUS MEDICAL PROBLEMS (like death...)Unless one is very experienced in pharmacology it is unwise to experiment with combinations of drugs. Even when using a single drug, thought should be given to all substances, both food and drug, which have been taken recently. Most primitive people fast or at least abstain from certain substances for several days prior to taking a sacrament. Substances most universally avoided are alcohol, coffee, meat, fat and salt. Some drugs potentiate others. For example, atropine will increase the potency of mescaline, harmine, cannabis and opiates. Many of the substances discussed in this book are MAO inhibitors. MAO (monoamine oxidase) is an enzyme produced in the body which breaks down amines and renders them harmless and ineffective. A MAO inhibitors interfere with the protective enzyme and leaves the body vulnerable to these amines. A common substance such as tyramine, which is usually metabolized with little or no pharmacological effect, may become dangerous in the presence of an MAO inhibitor and cause headache, stiff neck, cardiovascular difficulties, and even death. MAO inhibitors may intensify and prolong the effects of other drugs (CNS depressants, narcotic analgesics, anticholinergics, dibenzazepine antidepressants, etc.) by interfering with their metabolism. In the presence of an MAO inhibitor many substances which are ordinarily non-active because of their swift metabolism may become potent psychoactive drugs. The phenomenon may create a new series of mind alterants. However, because of the complex and precarious variables involved, it is risky and foolish for anyone to experiment with these possibilities on the non-professional level.
The most commonly used MAO inhibitors include hydrazines such as iproniazid, Marsilid, Marplan, Niamid, Nardil, Catron; also non-hydrazines such as propargylamines, cyclopropylamines, aminopyrazine derivatives, indolealkylamines, and carbolines. MAO-inhibiting materials discussed in this book include yohimbine, various tryptamines, especially 5-MeO-DMT and the methyltryptamines, and the various harmala alkaloids. The latter are especially potent inhibitors but, like yohimbine and the tryptamines, are short-lasting in action (30 minutes to several hours). Some of the commercial MAO inhibitors listed above are effective for several days to several weeks.
Among the materials which may be dangerous in combination with MAO inhibitors are sedatives, tranquilizers, antihistamines, narcotics and alcohol—any of which can cause hypotensive crisis (severe blood pressure drop); and amphetamines (even diet pills), mescaline, asarone, nutmeg (active doses), macromerine, ephedrine, oils of dill, parsley or wild fennel, beer, wine, cocoa, aged cheese and other tyrosine-containing foods (tyrosine is converted into tyramine by bacteria in the bowel).
SYRIAN RUE Peganum harmala. Family Zygophyllaceae (Caltrop family)

|
|
Material: Seeds of woody perennial native to Middle East. (Roots also active but seldom used.)Usage: 1 oz. seeds are thoroughly chewed and swallowed. Most effective when combined with other psychotropic materials, especially those containing tropanes.
Active Constituents: Harmine, harmaline and harmalol.
Effects and Contraindications: Hallucinogen; see harmine.
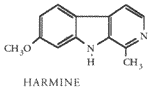
HARMINE 7-methoxy-1-methyl-9H-pyrido (3,4-b) indole.
Material: Indole-based alkaloid found in several plants including Banisteriopsis caapi (from which the South American hallucinogenic brew yage is prepared), Peganum harmala (Syrian rue), Zygophyllum fabago, and Passiflora incarnata (Passion flower).Usage: 25-750 mg harmine (see effects) is ingested on an empty stomach. In its hydrochloride form harmine may be snuffed (20-200 mg). Injection dosages are smaller: SC 40-70 mg; IV 10-30 mg. Absorbed poorly through stomach.
Effects: Harmine and related alkaloids are serotonin antagonists, hallucinogens, CNS stimulants, and short)term MAO inhibitors (100 x MAO inhibition of iproniazid but lasting only several hours). Small doses (25-50 mg) act as mild and therapeutic cerebral stimulant, sometimes producing drowsy or dreamy state for 1-2 hours. Larger doses up to 750 mg may have hallucinogenic effects, the intensity of which varies widely with the individual. Doses of 25-250 mg taken with LSD or psilocybin alter the quality of the experience of the latter. Telepathic experience have been reported with this combination.
Contraindications: Harmine is a brief MAO inhibitor. It should not be used with alcohol and certain foods and drugs (see list at end of file). When snuffed harmine may be slightly irritating to nasal passages. Large amounts may depress CNS. Since individual sensitivity varies this may occur with 250-750 mg.
Notes on other harmala alkaloids: Different harmala alkaloids vary in potency. The equivalent of 100 mg harmine is 50 mg harmaline, 35 mg tetrahydraharman, 25 mg harmalol or harmol, 4 mg methoxyharmalan. Harmal alkaloids are synergistic (mutually potentiating) and are therefore most effective when combined in an appropriate balance. Tropines (belladonna alkaloids) also potentiate harmals. Harmol and harmalol (phenols) in overdoses can cause progressive CNS paralysis.
Factors that change alkaloid levels in Phalaris
Increasing temperatures to 21C for day and 16C for night not only caused an increase in the proportion of alkaloids per weight, but it also increased the growth rate of the plant. Temperature regimes of 9c/4c and 15c/10c resulted in much less weight and fewer alkaloids. The data suggest that even higher temperatures may be better.Increased nitrogen supply in the plant's nutrients increased the plant alkaloids. Nitrogen content of solutions were 0.05, 0.5, and 5.0 times the nitrogen levels of "Hoagland's nutrient solution" (whatever that is). Just go with the principle "more is good."
Day length does not influence alkaloid levels.
Unfortunately, the data provided by the article do not specify how much the total increase in alkaloids were. However, examination of the data suggest the alkaloid content more than doubled as a result of the boosting treatments. Interestingly, the levels of 5-OH-DMT did not increase significantly!! Thus boosting the alkaloid levels appears to also decrease the relative concentration of this problematic alkaloid!
|
|
It has been whispered, in the dark and misty places in which people whisper these kinds of things, that maturity also influences that alkaloid levels of phalaris species. The maximum alkaloid levels were found during spring re-growth and in the seed shedding phase. At these times, alkaloids have been found to triple.
Another factor influencing alkaloid levels is genetic strain. Again, low alkaloid phalaris plants have been developed through breeding. Great for the sheep, bad for trip seeking humans. The seed you'll find available commercially will be low grade. Try to obtain seed from herb companies that cater to the psychedelic community.
Implications for Growing
To make the most of the light intensity factor, one might grow the plant in full sunlight/growlight until a few weeks before harvest, then reduce the light or shade the plant. I see no reason why alkaloid levels would not peak during this time. However, this technique would provide more mature phalaris, rather than the potent young stuff. With this method, one would have a large quantity of moderately potent phalaris. If one could shade outside plants, with plastic or fabric for example, this would make a great outdoor technique. If one were going to perform a chemical extraction, this would probably make the effort worthwhile by maximizing overall yield.Perhaps the plant could be kept in a re-growth phase by frequent clipping, like a lawn. In this case, one might grow a patch of phalaris in a wide shallow pot under grow lights. It should be allowed to grow up healthy and fill the pot under light that will maximize growth. Reduce light and harvest by clipping the plant down. Allowing the plant to grow large and healthy ensures that a good root structure will be built and will maximize re-growth. Turn light intensity up and allow the plant to put on new growth. After a short period of regrowth, reduce light, then clip. Repeat as desired. Because day length does not seem to influence alkaloids, the light/dark schedule should be set to maximize growth. I have no idea what ratio is needed. Of course the plants should be watered with a strong nitrogen fertilizer.
Remember, the research shows that the nitrogen, shading and temperature alkaloid boosts DO NOT increase 5-OH-DMT levels, resulting in a cleaner DMT / 5-MeO-DMT experience!
Preparation
The simplest way to prepare phalaris is to use a wheat juice extractor. This device presses the juice out of the leaves and stalks. Of course dosage becomes harder to gauge. One report on the internet suggested 1 teaspoon as a good dose and 2 teaspoons as an extreme overdose, resulting in one freaky trip. It appears likely, and has been suggested in some reports, that alkaloid levels drop when the plant dries. By taking the fresh sap, alkaloid levels should be maximized.Extraction of alkaloids can be performed on dry plant materials (based on Dr. Jonathan Ott's reports). An acid is used to exact the alkaloids of both the DMT and the MAOI containing plants. A solution of 1/3 lemon juice and 2/3 water is used to quickly boil the plant material. Pour off and repeat two more times. The dose is easier to determine because it is based on dry plant material. Three grams of Syrian rue per dose of DMT provides the required MAOI. Again, increasing the dosage of Syrian rue beyond 3g does not increase the potency of the DMT significantly more than 3g.
Important note: I have always seen references to staying away from aluminum for all sorts of chemical extractions and procedures. I believe that this is because aluminum bonds with a lot of contaminants that can then find their way into the brew (just think about having to clean calcium deposits off of your aluminum coffee maker with vinegar). Always use nice glass (pyrex is much less likely to break) or stainless steel.
The liquid form can serve as an oral dose, or it can be evaporated gently and slowly to a goo. This goo can then be smoked for a very intense dose of DMT. Or the goo can be saved for oral doses. I recommend refrigeration. I have no information as to how to best store phalaris products to prevent spoilage and loss of potency.
There are a variety of chemical extraction techniques floating around on the net. I have little ability in evaluating the accuracy and safety of these techniques. I prefer to speak on subjects in which I have some faith in my knowledge. In any case, it does not seem likely that for small time experimenters, chemical reductions to pure alkaloids will be necessary. The above methods should work.
In closing
This article, as noted in opening, is probably not totally accurate or complete. If new or more accurate information arises, or if a topic has been missed, I would appreciate it if new chapters are written, and notes are made. Please type in the text "(See note ##)" and add your note to the END of the text. Stick new chapters where appropriate. I consider this a public work. Be accurate and cite your sources!Unanswered questions
- Why are human recreational doses of DMT fatal to sheep?
- What day/night schedule will maximize phalaris growth?
- What other plants are potentially useful?
- How should one best preserve phalaris/DMT?
- Is 5-MeO-DMT also a MAOI?
References
- Baxtor, C. & Slaytor, M. (1972). Phytochemistry, 11, pp.2767-73.
- Gessner, P K. (1970). In D. H. Efron (editor) Psychotomimetic Drugs. Raven Press.
- McLoed, W. R., &Sitaram, B. R. (1985) Acta Psychiactrica Scandinavica, 72, 447-450.
- Moore, R. M., Williams, J. D., Chia, J. (1967). Australian Journal of Biological Science, 20, 1131-40.
- Oram, R. N., Williams, J. D. (1967). Nature, March 4, pp.946-7.
- Ott, J. Ayahuasca Analogues: Panthaen Ethnogens. 1994. Natural Products Co.
- Shulgin, A. T. (1981). Journal of Psychoactive Drugs, 13(4), 389.
deoxy hypertext last update : Nov. 25, 2002
Smokable Dimethyltryptamine from Organic Sources
deoxy > hyperspace
> DMT